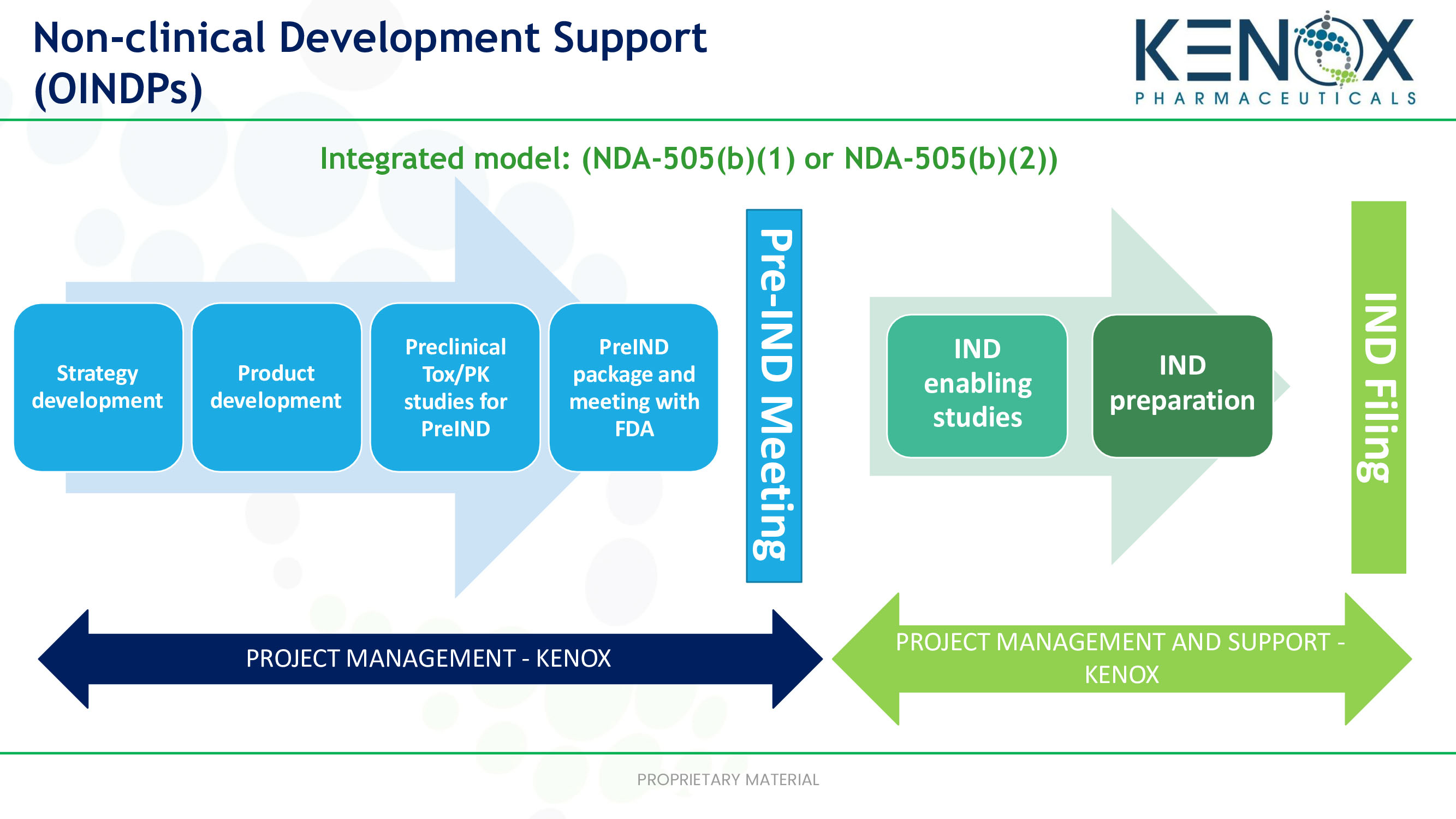
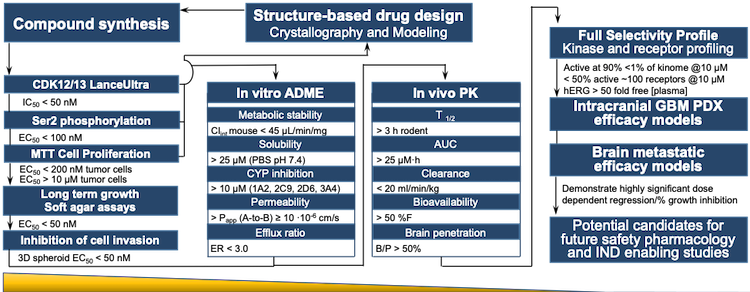
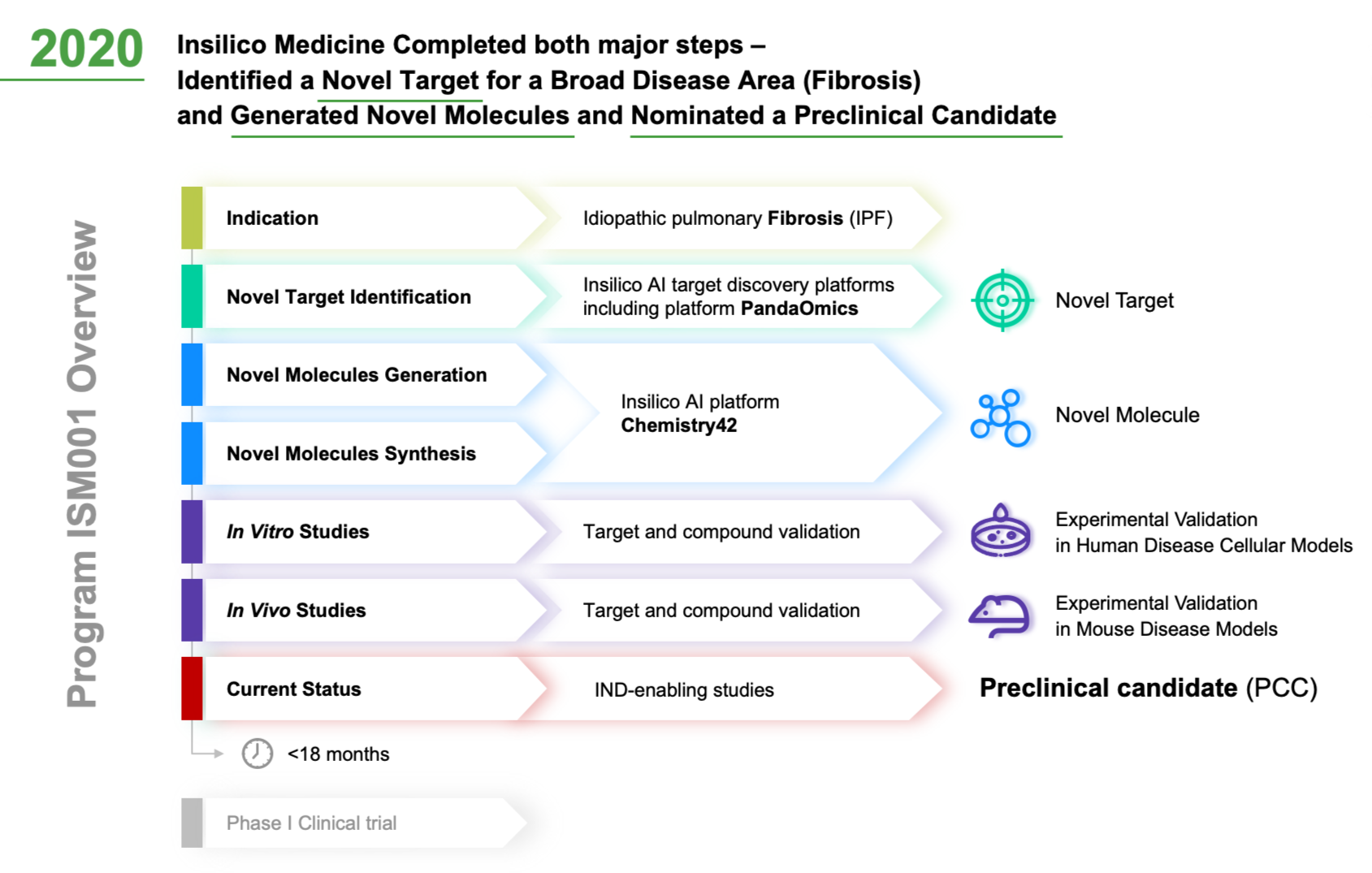
Applied StemCell Inc on X: "ASC can develop preclinical assays to determine efficacy, pharmacodynamic, dose-ranging studies, biodistribution, and develop other IND-enabling assays to measure potency and toxicity of the drug including NHPs,

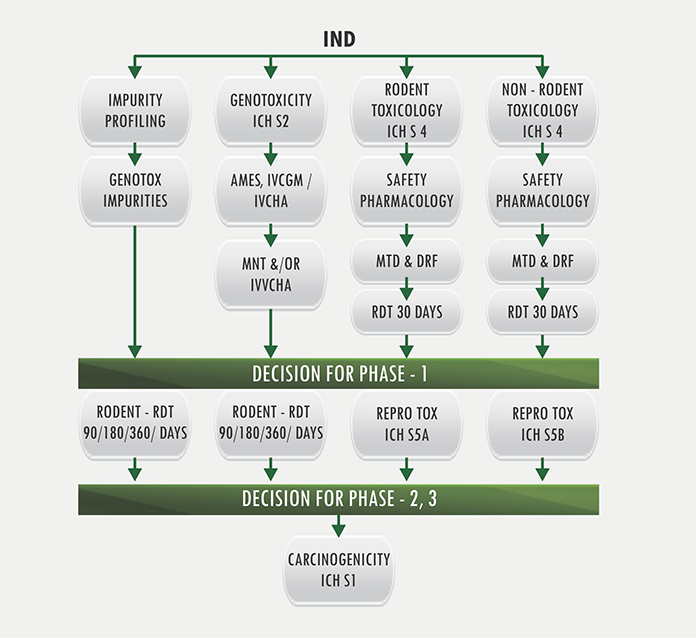
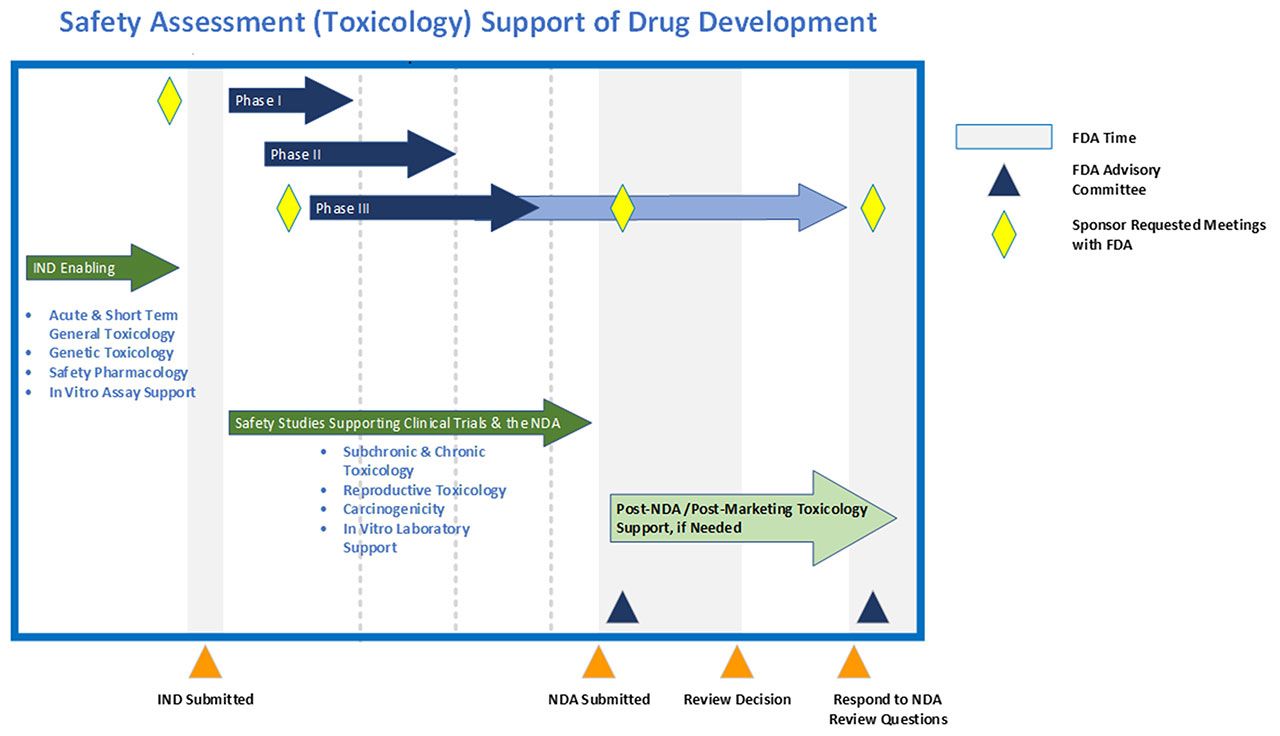
The alignment of major PK/PD related studies with the decision points... | Download Scientific Diagram
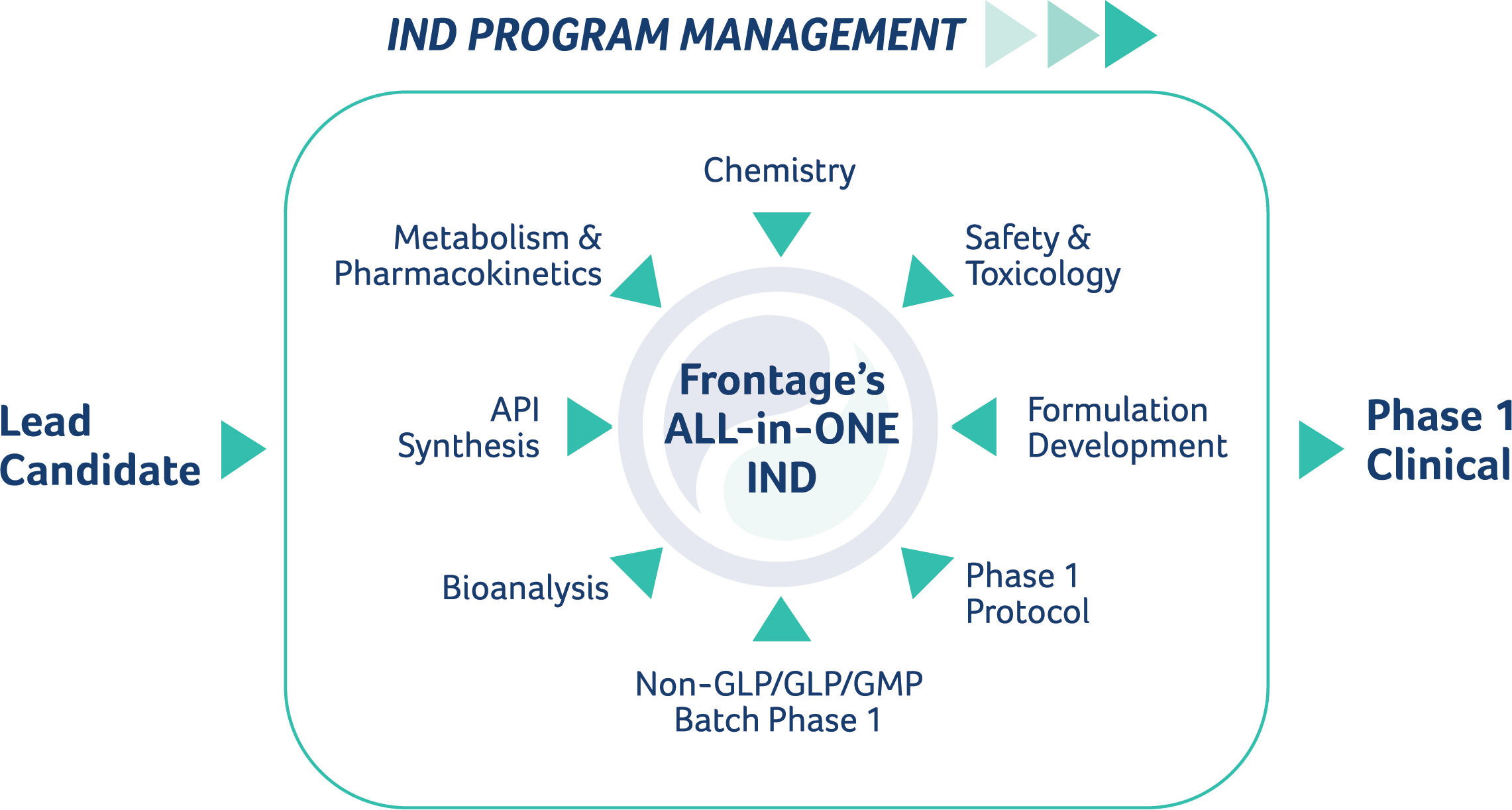
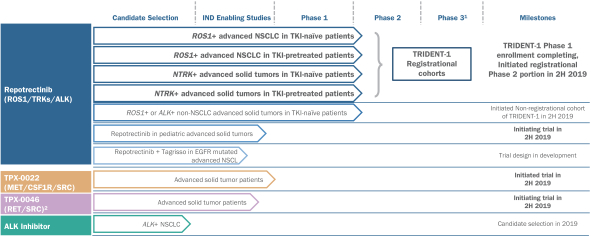
PepGen Announces IND-Enabling Preclinical Data Supporting Progression of PGN-EDODM1 into Clinical Studies | PepGen